Regorafenib for Gastrointestinal Stromal Tumors
Third line treatment of GIST
Obligatory—this is not medical advice
It’s rare in the treatment of sarcomas that there’s a defined path. For diagnoses, such as malignant peripheral nerve sheath tumors, there are loose guidelines to which a patient and a physician might hew. There are always exceptions, of course, but, large and by, the landscape of treatment for patients with most sarcomas past the second line of systemic therapy is a little muddied. This is why being honest and up front about what we know is so important.
For most patients with GIST, however, there is an established order. Medications have been tested in sequence. Studies started with imatinib and were followed by sunitinib, regorafenib, and ripretinib. New medications are also being evaluated for later lines, as well as to augment other treatment. We continue to improve and it may very well be that advanced GIST becomes one of the first solid tumors that is a true chronic disease. Many sarcoma specific oncologists will tell you anecdotes of patients who have been on imatinib since the inaugural clinical trials (20 years or more). We need more medications that can provide durable control and even cure our patients.
Regorafenib
Regorafenib exists within the family of medications called tyrosine kinase inhibitors(TKIs), a subclass of targeted therapy. It is important to understand that, in its being a distinct molecular entity, regorafenib has a different spectrum of activity when compared to other TKIs such as imatinib or sunitinib.1 In addition to hitting proteins that are important for cancer growth, it also has ‘off target,’ or deleterious effects on proteins expressed in normal cells.2 The balance of on-target and off-target effects ultimately determines the utility of a TKI.
As seen above, regorafenib has the ability to abrogate VEGF, c-KIT, RET, and other proteins. The relative capacity of it to do this, while also being tolerable, is what has led to its approval for multiple indications by the FDA.3
Regorafenib for patients with GISTs
The clinical trial which has most often been cited as leading to the FDA approval of regorafenib for patients with GISTs is the GRID study.4
Study Design:
Phase 3 trial done at 57 hospitals in 17 countries in patients with advanced GISTs. Patients must have been failed by imatinib and sunitinib.
Enrolled patients were randomized 2:1 to receive regorafenib 160mg daily or placebo, each with best supportive care. This was done in a double-blind fashion. The cycle structure for regorafenib was 21 days on the medication and 7 days off with a total cycle duration of 28 days. This had been normal for other trials at the time and is secondary to tolerability of regorafenib.
The primary endpoint was progression free survival with secondary endpoint of overall survival. Patients were allowed to crossover to open-label regorafenib (when failed by placebo they could receive regorafenib).
Nuances:
50% of patients must have received fewer than three prior types of treatment for GIST
The fact that patients failed by >3 types of treatment were allowed to enroll is uncommon in other modern trials evaluating patients at a specific line of treatment. One could make the ideologic argument that limiting would bias the population and that third line was not yet approved, but, we should still recognize that the population was not restricted wholly.
Progression free survival was assessed according to RECIST v. 1.1 and previously irradiated lesions were allowed so long as there was evidence of progression in that lesion
Performance status of 0 or 1 was required (new studies allow for ECOG of 2, as the disease can limit patient condition)
Patients were required to be off of TKI for 7 days in the case of imatinib and 10 days in the case of sunitinib
PFS was assessed by blind central review
Mutational status was not known prior to enrollment
Crossover was frequent (more on this later)
Overall, I would remark that enrollment criteria were fairly reasonable. I cannot say they’re perfect, but no trial is.
Results
240 patients ultimately were screened with 199 patients randomized (133 regorafenib, 66 to placebo). Of 41 patients excluded, 29 did not meet inclusion, 3 had AE, 4 died, 5 withdrew consent. Of those on placebo, 56 crossed over to open-label regorafenib (56/63 eligible to do so ~89%), which is very good.
At the time of final analysis, 81 of 133 patients (60.9%) in the regorafenib group and 63 of 66 patients (95.5%) in the placebo group had progression by RECIST criteria. Patients in the regorafenib group had a median treatment duration of 22.9 weeks. The mean daily dose for patients was 139.8mg. Notably, in the regorafenib group, patients received 78% of the planned dose, while patients in the placebo group received 83.8% of the planned dose.
Median progression free survival was 4.8 months in the regorafenib group and 0.9 months in the placebo group (HR 0.27, p<.0001). Investigator assessed was also significantly higher, although not the primary endpoint. There were few responses by RECIST criteria, response rate of 4.5% in the treatment group and 1.5% in the control group. The overall survival difference was not significant (eg patients did not live longer by starting regorafenib earlier).
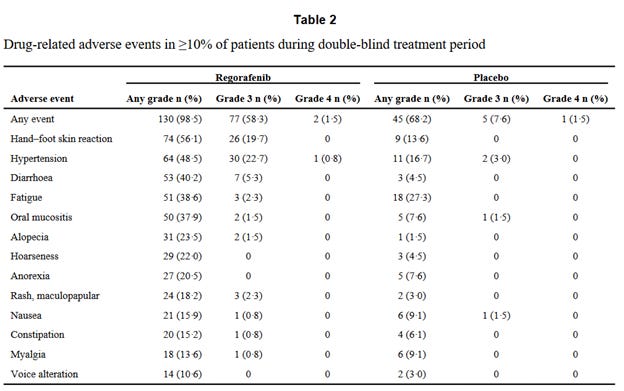
All patients receiving regorafenib experienced adverse events. 61 of 66 patients in the placebo group also had adverse events. See the table below for specific incidence of each. Importantly, grade 3 drug related adverse events were seen in 61.4% of patients in the regorafenib group—this is not a sugar pill. The most common were high blood pressure, hand-foot reaction and diarrhea. Dose reductions happened in 72% of patients as a result of this.
Because of the relative difficulty in tolerating regorafenib at 160mg alternative dosing schedules have been proposed in other solid tumors. 5
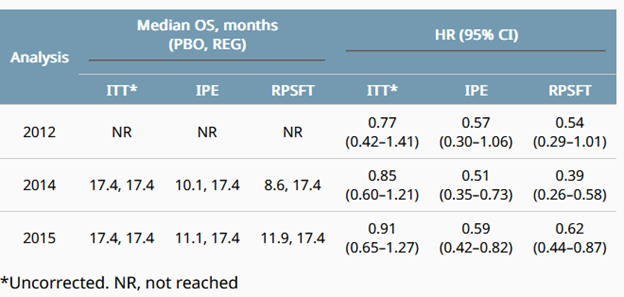
The final overall survival analysis for this trial were reported in 2016. 6There was not a significant difference in survival, and, understandably, this was attributed to crossover. See the IPE column for computed incremental survival benefit. While I cannot speculate as to the accuracy of this, it is more than likely that being on *some* form of TKI is beneficial for patients with GISTs. We just have not measured the survival difference.
Avapritinib vs Regorafenib for Third Line Treatment
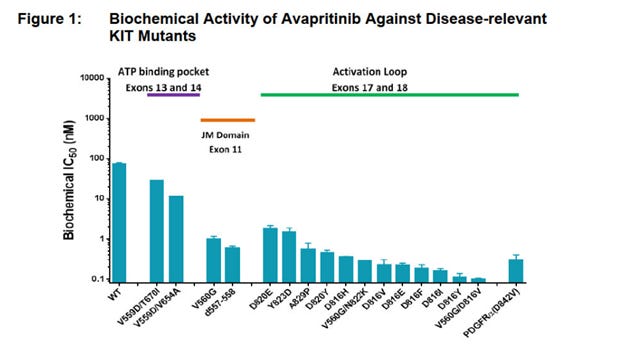
Given the relative toxicity of Regorafenib and lack of measured survival benefit in the initial intention to treat study, challengers have arisen. In 2021, the results of the VOYAGER trial of avapritinib vs regorafenib was published in the journal of clinical oncology.7 Avapritinib is another TKI (see below image from the protocol).
The VOYAGER trial was a randomized, phase 3 study performed internationally in North America, Europe, Australia, and Asia. Patients were assigned 1:1 to regorafenib or avapritinib. This was stratified by TKI treatment (third-line or fourth-line), PDGFRA D842V status, geographic region of residence. The dose of avapritinib was 300mg based on phase I data from the NAVIGATOR Phase I trial. 8
This was a trial designed to measure the superiority of avapritinib. The primary endpoint was progression free survival as measured by mRECIST v 1.1 (modified RECIST). 9 Secondary endpoints were response rate and overall survival. Exploratory endpoints looked at mutations, cognitive function (known side effect of avapritinib), and patient reported outcomes.
The inclusion criteria were as would be expected. Patients must have received imatinib and 1 or 2 other TKIs in treatment of their GIST, including TKIs used after resection. Patients must have disease progression prior to enrollment. Performance status was 0 or 1. Patients who had received either treatments in the trial were excluded as were patients with wild type GISTs. Other exclusion criteria were standard practice for phase 3 studies (poor organ function, etc). Crossover was allowed.
476 patients were randomly assigned between treatment arms. Importantly, there was no significant difference in modified progression free survival. Numerically, regorafenib had a better mPFS than avapritinib.
As one can see, avapritinib did not show superiority in this trial when it came to the primary endpoint.
The frequencies of adverse events were similar between groups with frequency being 92.5% in the avapritinib group and 96.2% in the regorafenib group. See above for the specific breakdown.
I apologize if I’ve somewhat skimmed over avapritinib here, but I do very much feel that its discussion is kind of within the regorafenib environment of this article—and therefore should be limited as such. There are some real benefits to this medication as demonstrated in the NAVIGATOR trial for patients with PGGFR D842V mutations, and this can be reviewed separately. It’s worth granting it more time.
Conclusions
Regorafenib has been shown to improve progression free survival (time until growth of tumor by imaging criteria or death) vs placebo. Post hoc studies may indicate an overall survival benefit, again when compared to placebo. It remains the standard of care for most patients with GISTs in the third line. A large phase 3 trial comparing avapritinib to regorafenib, the VOYAGER study did not show superiority of avapritinib. Regorafenib has many side effects and therefore careful and judicious consideration should be given to whom receives this medicine. There will be future studies in this space that will determine which patients are most likely to benefit from what treatment. Next-generation sequencing, or analysis of the underlying driver mutations for a patient’s GIST will likely be the backbone of such trials. There continues to be a tremendous need for tolerable and effective medications for our patients with advanced GISTs.
https://pubmed.ncbi.nlm.nih.gov/22577890/
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203085s007lbl.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3819942/
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30272-4/fulltext
https://ascopubs.org/doi/abs/10.1200/jco.2016.34.4_suppl.156
https://ascopubs.org/doi/full/10.1200/JCO.21.00217
https://www.ejcancer.com/article/S0959-8049(20)31423-4/fulltext
https://pubmed.ncbi.nlm.nih.gov/24951349/









Thank you for sharing your knowledge with the sarcoma community.
I appreciate the hard work you put in to get this newsletter to us in the Sarcoma community. Your humanity shines on in every article that you write. Thank you,
Manuel Salinas GIST patient stage 4