Obligatory—this is not medical advice
Previously, we reviewed the role of imatinib for patients with a diagnosis of GIST. This medication is administered both in the adjuvant as well as the advanced setting. As beneficial as imatinib can be for the treatment of advanced and unresectable disease, many patients will eventually need other forms of treatment. The mechanism for resistance to imatinib can vary and probably warrants consideration. Resistance mechanisms are sometimes elucidated by genetic sequencing. Most patients, however, will be best treated with sunitinib, which is FDA approved for the management of advanced GIST refractory to imatinib.
What is sunitinib?
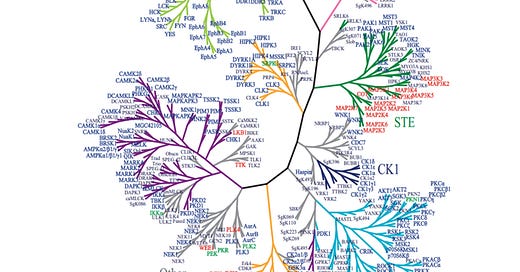
Sunitinib, like imatinib, is a tyrosine kinase inhibitor. Protein kinases play important roles in many cellular processes. Various kinases can have abherrent expression in many different types of cancer. There are multiple kinases known to be involved in the development and continued growth of GISTs. The relationship of these kinases to one another is represented below in the ‘kinome.’1
While there are clearly many proteins in the body, only some are impacted by the medications we prescribe. This bar graph indicates relative inhibition of different proteins by sunitinib. Note that sunitinib act on KIT, PDGFR, FLT3, FGFR, PDGFR, amongst other targets. The success of this medication against the cancers for which it is prescribed theoretically depends on its ability to abrogate, or block, the action of these protein kinases, while also not interfering with the function of normal cells or causing an undue burden of side effects for patients.
Sunitinib for treatment of GISTs
Sunitinib was approved by the FDA in 2006 for patients with advanced GIST whose disease was refractory to imatinib. This was based on the results of a trial published in 2006. 2
Trial characteristics:
Randomized, blinded clinical trial of 312 patients
Patients were randomized 2:1 to receive sunitinib vs placebo.
Dosing of sunitinib was 50mg daily for 4 weeks, followed by a 2 week period off of treatment for a total of a 6 week cycle.
The primary endpoint was tumor progression as determined by imaging (RECIST criteria). Secondary endpoints included progression-free survival, overall survival, response rate, amongst others.
Patients assigned to placebo were allowed to cross over to sunitinib at time of progression.

The results of this clinical trial indicated that the median time to tumor progression was 27.3 weeks in patients treated with sunitinib, and 6.4 weeks in patients who received placebo. A secondary endpoint of overall survival also suggested benefit for sunitinib with a median overall survival not reached in the sunitinib group and a significant hazard ratio.

These data indicated that not only was sunitinib beneficial from a tumor control standpoint, but also that patients who were initiated on sunitinib first, as opposed to placebo, lived longer. This led to the approval and standardization of sunitinib as a medication administered in the second line, after failure of imatinib for most patients with GISTs.
Although sunitinib was efficacious, there was a relatively high rate of adverse events or side effects. 83% of patients reported at least one treatment-related adverse event compared to 59% of those receiving placebo. 20% of patients who received sunitinib had a serious adverse event. As a result of this, dose reductions or treatment interruptions occurred in 9% of patients receiving sunitinib.
Recent Sunitinib Trials
Since 2006, multiple medications have been FDA approved for later line therapy for GIST, including regorafenib (3rd line), and ripretinib (4th line) with their own, placebo controlled trials. Understandably, questions arose about whether, given the success of these medications in later lines, there may be benefit to introducing them earlier, or in lieu of sunitinib. The INTRIGUE trial attempted to discern whether ripretinib was superior to sunitinib in the second line.3
Trial details:
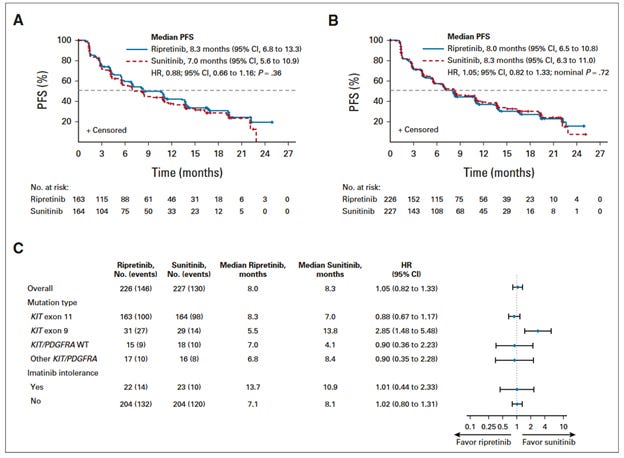
453 patients were randomly assigned to ripretinib or sunitinib in a 1:1 fashion.
These patients had to have had archival tissue indicating a KIT or PDGFRA mutation by DNA sequencing.
The primary end point was progression-free survival by independent radiologic review using RECIST criteria.
This was tested with intention-to-treat in populations of patients with KIT exon 11 mutations, and all participants.
The primary endpoint of this clinical trial, improved progression free survival (a composite endpoint of growth of the tumor by 30% at any point, or death), was not met. Ripretinib did not demonstrate a statistically significant improvement in PFS over sunitinib. 55.2% of patients receiving sunitinib had grade 3 or higher adverse events, while these occurred in 26.5% of patients taking ripretinib. 38% of patients in the ripretinib arm, compared to 63.3% of patients in the sunitinib arm needed dose modification or interruption.
Subsequent analysis of this clinical trial looking at circulating tumor DNA highlighted differential response of patients with various resistance mutations. These are hypothesis generating, indicating that ripretinib may have increased efficacy in patients with exon17/18. 4
These data may provoke future prospective trials to further answer the question about which tyrosine kinase inhibitor is beneficial for patients with specific resistance mechanisms.
Conclusions
At the time of this writing, data indicate that sunitinib remains the appropriate choice for the majority of patients with GISTs who have been failed by imatinib in the advanced setting. Nonetheless, future clinical trials may provide additional data and help us determine which patients are most likely to benefit from each approved treatment. It is highly likely that following a rote sequence in all cases is suboptimal, but conclusive data are required prior to changing the standard of care.
https://onlinelibrary.wiley.com/doi/full/10.1111/gtc.12022
https://www.thelancet.com/journals/lancet/article/PIIS0140673606694464/fulltext
ascopubs.org/doi/abs/10.1200/JCO.22.00294
https://ascopubs.org/doi/pdf/10.1200/JCO.2023.41.36_suppl.397784?role=tab