Olaparib and Temozolomide
Later Line Treatment of Uterine LMS
Obligatory—this is not medical advice
The treatment of leiomyosarcoma (LMS) has made strides in the first line setting.1 While data from LMS-04 indicate increased rates of response, as well as progression free survival (PFS), we are still awaiting overall survival (OS). Other clinical trials within this space have had varied results, often relying on treatment with relatively toxic agents and showing possible benefit measured in weeks. 2 A recent JCO article, published by Ingham et al. is notable, particularly in light of its preceding an eventual Phase III study.3
Study Background
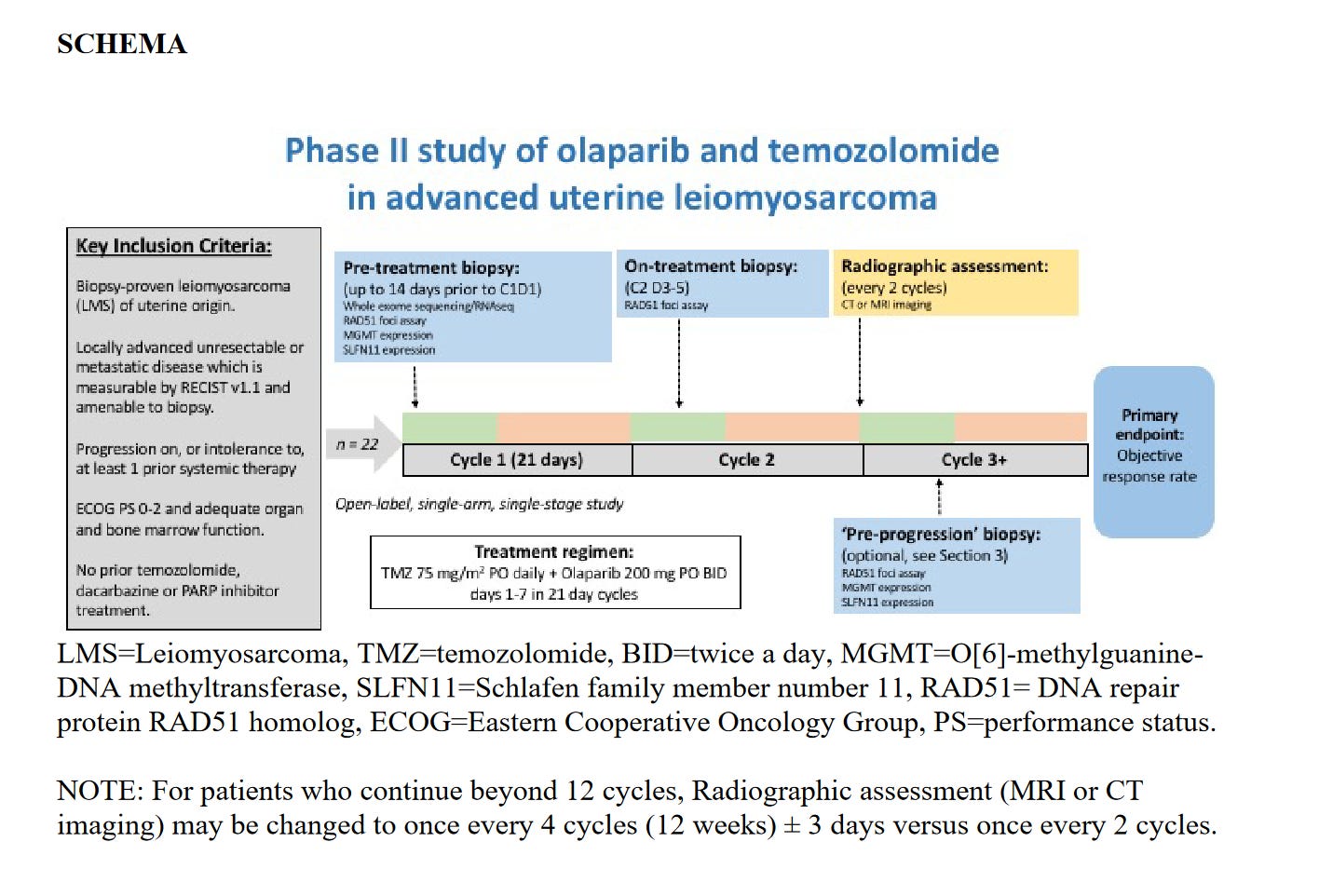
NCI Protocol 10250 was a Phase II study of olaparib and Temozolomide in patients with advanced (eg metastatic or unresectable) uterine LMS (uLMS). The full protocol is available.4
Notable Inclusion/Exclusion Criteria:
Advanced uLMS
Performance status <= ECOG of 2
Progression after at least one prior systemic treatment (adjuvant did not qualify)
NO UPPER LIMIT
Exclusion: Prior PARP or temozolomide or dacarbazine exposure (dacarbazine had historically been used in earlier lines, but I would say this has fallen out of favor)
Exclusion: involved in planning and/or conduct of study (? assuming 2/2 COI, but seems unecessary)
One thing I would note is that the screening procedures were required within 14 days (this is very short). In terms of coordinating a biopsy, imaging, etc this will select for centers with availability and patients capable of doing these tasks in a short timeframe. Normal screening windows are 28 days.
Assuming that you were enrolled, you would receive:
Temozolomide 75mg/m2 on days 1-7 of a 21 day cycle (can be 75mg/m2 daily for STS off label)5
Olaparib 200mg BID for days 1-7 of a 21 day cycle (normally 300mg bid continously for FDA approved indications)
These are dose reduced for overlapping AEs.
Results
Patients who enrolled were median age of 55, ECOG PS 0-1 (no PS of 2 probably secondary to the entry requirements as per above), and 59% had >3 lines of treatment. Surprisingly few had gotten Trabectedin, even though this was FDA approved prior to enrollment, which commenced in 2019—that stated, there was no OS benefit per Demetri et al.6 I would also say it’s surprising how little doxorubicin exposure there was, with prior study results.7 86% of patients had received gemcitabine and docetaxel.
23% of patients had an objective response by RECIST. Viewing the spider plot, some of these were sustained out past 30 months. Correlative analyses were performed, but the numbers were too small—so this is entirely speculative. RAD51B, PALB2, and ATR ‘achieved best response of SD and PFS of 31.5, 27.3, and 7.1 months, respectively.’ More study is needed before coming to conclusions here. Dissecting out the homologous recombination deficient specimens showed that those patients found to have deficiency had an increased PFS, again small numbers and hypothesis generating.
So how toxic was it? Essentially all patients experienced some grade of hematologic toxicity (these are seen with either drug alone, so not surprising). It was wise to reduce the doses of olaparib and temozolomide. Even so, 36% of patients needed at least one dose reduction of olaparib. 45% of patients needed one dose reduction of temozolomide. Three patients stopped treatment secondary to toxicity. This rate of discontinuation is less than more toxic combinations, but still not insignificant.8
Closing Thoughts
I want to commend the authors for this study. As far as phase II trials are concerned, this is an impressive feat with correlatives and conducted in what appears to be a very exacting way. Furthermore, the endpoint of ORR is an appropriate endpoint for Phase II studies, and, in my opinion, superior to the increasingly common progression free rate at 12 weeks. The authors are taking the right next steps and moving into a randomized phase II/III clinical trial comparing to active control in the second line (trabectedin or pazopanib). It will be exciting to see the results.
https://pubmed.ncbi.nlm.nih.gov/35835135/
https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.11505
https://classic.clinicaltrials.gov/ct2/show/NCT05633381
https://ascopubs.org/doi/pdf/10.1200/JCO.23.00402?role=tab
https://pubmed.ncbi.nlm.nih.gov/16134177/
https://pubmed.ncbi.nlm.nih.gov/26371143/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5622179/
https://pubmed.ncbi.nlm.nih.gov/34050255/