Obligatory—This is not medical advice
The treatment of sarcoma is always multidisciplinary. Every patient at time of diagnosis merits review by a team of medical oncologists, surgeons, radiation oncologists, pathologists, radiologists, and others. There may be cases where we need to wait on information for a clearer picture, for instance if imaging or a biopsy result is pending prior to tumor board. Nonetheless, an ample tumor board discussion is merited to ensure that we’re utilizing all of resources as best as possible for each patient. These meetings are times where we can also contribute our individual knowledge based on readings, or prior cases, to the discussion and grow as a group. It’s what forms the bedrock of a successful academic center and continues to be an emphatic strength of UCSF and other sarcoma centers. It’s why it’s so important that patients be evaluated at a sarcoma center, even if in many instances it does not alter management. Sometimes it does.123
Combined Chemotherapy and Radiation
The track record of chemotherapy combined with radiation, or chemoradiation, for high-grade soft tissue sarcomas of the extremities has been mixed. The most robust data is from Radiation Therapy Oncology Group (RTOG) 9514. This was published in 2006 and is available online. 4 Here are some summarized details:
This was a phase 2 trial designed to assess safety and efficacy of administration of interdigitated (interspersed) radiation and chemotherapy
Total dose of radiation was 44 gray
Chemotherapy was Mesna, Adriamycin, Ifosfamide, and Dacarbazine (MAID)
Doxorubicin (Adriamycin) 20mg/m2/day for 3 days
Ifosfamide 2500mg/m2/day for 3 days
Dacarbazine 225mg/m2/day for 3 days
Mesna 2500mg/m2/day for 4 days
66 patients were enrolled, 64 analyzed
3 patients DIED from treatment
53 patients had life threatening toxicity (not including those who died)
Without going into too many details, the conclusions were that this regimen was too toxic to be broadly applied. 91% of patients had resections with negative margins, although 5 of these were amputations. At a median follow-up of 2.75 years, 14 of 64 patients had died. Modern neoadjuvant studies for chemotherapy and radiation alone have not had treatment related mortality. 5 While yes, the regimens are different, more toxicity does not necessarily mean better—we should do well to remember that. 6
Combined Trabectedin and Radiation for patients with Myxoid Liposarcoma
The underlying rationale for this combination is the following:
Trabectedin is relatively well tolerated as a single agent treatment with high numerical rates of efficacy for patients with myxoid liposarcoma. 7
Myxoid liposarcoma is likewise sensitive to radiation with large reductions in tumor volume compared to other types of sarcoma after neoadjuvant dosing (50Gy)8
These treatments were therefore combined.9 So, what did they do exactly? This was an open-label, phase 2 nonrandomized clinical trial that enrolled in Europe. Patients needed to have disease that could be removed by surgery, or resectable, but was still exceeded a size threshold. All grades were taken. See how this could have led to wide ranges of future outcomes here. Patients received standard dosing of trabectedin, which was 1.5mg/m2 on day 1 of each 3 week cycle (21 days). This is a 24 hour continuous infusion. Radiotherapy was started on day 2 of treatment with a total of 25 fractions given and a total dose of 45 Gy. The primary outcome was overall response by RECIST version 1.1.10 This endpoint may be used for efficacy, but ultimately does not answer the questions most pertinent to patients, that is recurrence risk or survival. A treatment success rate of 70% (indicating responses in 70% of patients to this treatment) was used as the threshold against which a null hypothesis of response rate of 50% was tested.
In total, 46 patients were recruited to the phase 2 portion of this trial. I would say that their demographics are similar to those reported for patients with myxoid liposarcoma (reference prior liposarcoma article). Rate of low vs high grade was approximately 50/50.
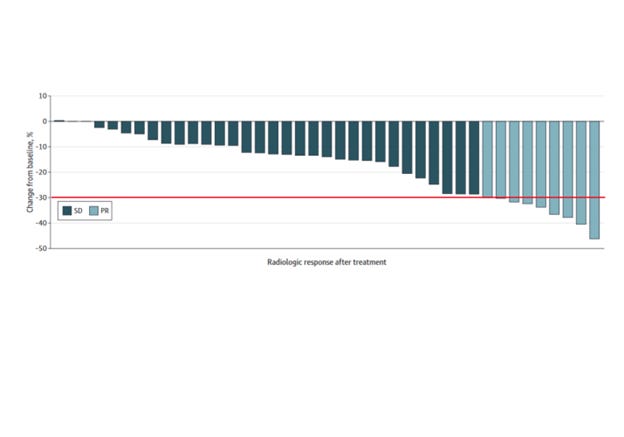
Above, you’ll see the results by RECIST response. 8 of 42 patients had a response by these criteria, indicating a 19% response rate. The rest had stable disease. While not a primary end point, CHOI criteria, an alternate imaging criteria, was assessed in 29 cases.11 The partial response here was 83%. I would remark that the significance of this within this particular histologic subtype is unknown, and, insofar as not all patients underwent this form of analysis, we do not know if there was some form of selection bias at play.
More intriguingly, for 39 of 42 patients whose tumors were analyzed, the median viable residual tumor was 10% with 20 of 39 of those patients having a less than 10% viable tumor. At a median follow-up of 37 months, 7 of 42 had recurrence. None had died. The rest of the details regarding numbers reported may be seen in the paper.
How do we interpret these data?
Firstly, I commend the investigators on recruiting this portion of patients to undergo this intervention and adhere to established criteria. The conclusions are highly reasonable as described—that while this is tolerable, we cannot make a blanket recommendation for its application. Further, and perhaps, more directed evidence is needed.
The sarcoma literature has been plagued with trials whose findings are nebulous (see EORTC 62931).12 Our answers are only as good as the strength of our questions. Neoadjuvant data published after initiation of this study seem to indicate equipoise in high grade myxoid liposarcoma for administration of trabectedin vs anthracycline and ifosfamide. It is therefore possible that the population of patients undergoing this intervention was not properly constrained to those with high grade myxoid liposarcoma, and so the treatment effect was diluted. Reading the manuscript, and scanning the supplement, I was unable to find the rate of response by grade of the sarcoma at time of diagnosis. This could have been useful information from a hypothesis generation standpoint.
In short, we still have some remaining questions:
Is there a difference in efficacy for this treatment between high and low grade myxoid liposarcomas?
Is there any advantage in giving combined chemoradiation as opposed to in sequence?
Might alternative administrations of trabectedin remain effective in this or other settings (precedence for 3 hour adminsitration)?13
Is RECIST the most appropriate criteria to judge efficacy?
Coud short course radiation be combined with trabectedin?14
Clearly, there are more.
Conclusions
Neoadjuvant trabectedin and radiation for myxoid liposarcoma remains something that, while technically feasible, should only be performed or suggested under the direction of an experienced sarcoma center. There are many lingering questions about these data and how they can be applied to patients with localized myxoid liposarcoma. We need to continue to push to answer these questions for our patients as well as seek as meaningful endpoints as possible.
https://pubmed.ncbi.nlm.nih.gov/31081028/
https://pubmed.ncbi.nlm.nih.gov/9842949/
https://ascopubs.org/doi/abs/10.1200/JCO.2005.02.5577?role=tab
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(17)30334-0/fulltext
https://www.nejm.org/doi/full/10.1056/nejmoa030684
https://www.annalsofoncology.org/article/S0923-7534(19)34435-7/fulltext
https://www.redjournal.org/article/S0360-3016(04)00462-6/fulltext
https://ctep.cancer.gov/protocoldevelopment/docs/recist_guideline.pdf
https://pubmed.ncbi.nlm.nih.gov/17470865/
https://pubmed.ncbi.nlm.nih.gov/35835135/
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(22)00638-6/fulltext