Obligatory - This is not medical advice
This last week was particularly exciting for me. Although I’d been tasked with attending the inpatient oncology service while also maintaining my normal day to day duties (outpatient clinic, medical directorship, whatever other ring I put my hat into), we finally have the publication of the Defi trial.1 In a sentence, this was a placebo controlled, randomized, double-blind study evaluating the merits of nirogacestat (gamma-secretase inhibitor) in treating patients with desmoids. It was positive. Let’s talk about it here.
For more information on what a desmoid tumor is, see my recent post.
How do we treat desmoid tumors?
There has been significant variance in clinical practice for treating desmoid tumors. As I’d touched on earlier, most patients undergo surveillance initially. Depending upon symptoms, discussion, imaging, etc, we may elect to start treatment. Within this group, there is a bevy of options, but only a select few have been prospectively studied—namely sorafenib and pazopanib. There are still some unanswered questions here, but nonetheless, these are acceptable front line medications. 23
I’ll delve into the sorafenib trial in more detail elsewhere, but suffice it to say, despite its toxicity and price, it has been the standard of care for most patients—and consequently the oncology board answer for first line treatment. It’s not an easy medication to administer with significant monitoring requirements. Patients know they’re on it. This is generally true of other treatments we use for patients with desmoid tumors, as well, such as pazopanib, methotrexate, vinblastine, liposomal doxorubicin, etc.
This is not me saying that nirogacestat is without toxicity (clearly that’s not the case, as we’ll see). It’s just to say that we need frame where we are. I’ll focus on sorafenib here so that we can understand a little better.
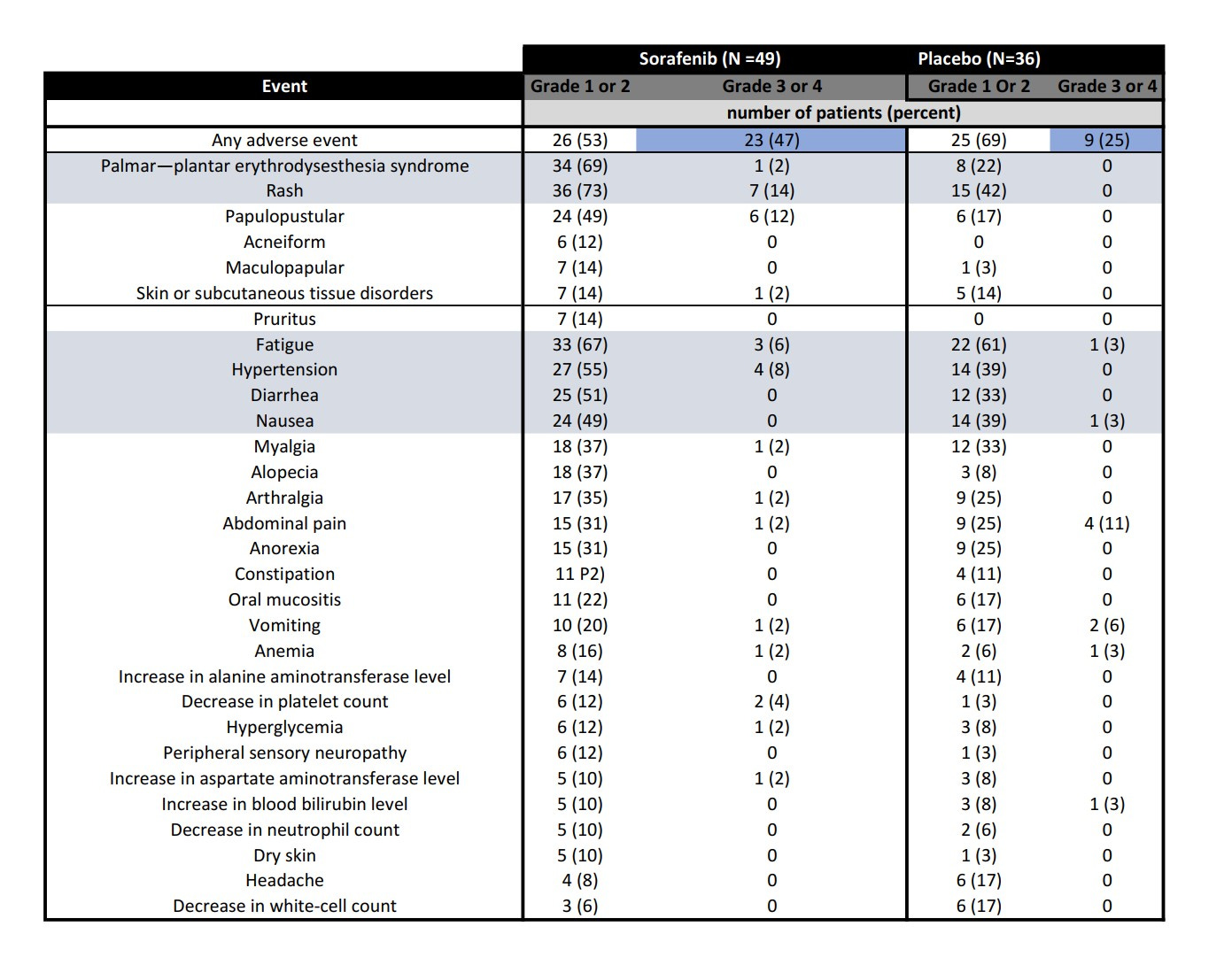
The dark blue indicates the relative incidence of grade 3 or 4 (read: SERIOUS) adverse events by CTCAE v 4.0. For instance, grade 3 fatigue means that someone is excessively tired to the extent that they can no longer care for themselves, and it is not relieved by rest. Rashes would cover >30% of the body and possibly limit self care as well. The burden these symptoms have on patients is belied by these figures. 20% of patients stopped the medication. As you can imagine, this is a particularly motivated group of patients who generally are healthier and have more resources, and therefore the ability to participate in trials.
So, we have a toxic therapy, that much is a given. How about efficacy? Well, it proved that as well.
The progression free rate, or the percent of patients who did not have their tumors grow, was 81% in the sorafenib group and 36% in the placebo group. The response rate was also higher, but, still approximately 20% in the control group. That means that 20% of patients who did not get active treatment still had their tumors shrink. I can delve more into the details in a separate post, but this gave oncologists a tool that would halt growth in the vast majority of patients. There were tradeoffs, but it was an option for patients.
We need to know where we are to put this all into practice. So, moving forward from our knowledge of Sorafenib, and the publication from 2018, let’s take a look at the DeFi trial.
Alright, so what is nirogacestat?
Nirogacestat is a gamma-secretase inhibitor. What does that mean? It inhibits a collection of membrane-associated proteases from doing their job. The part that we're particularly interested in is that it abrogates parts of the Notch pathway. Notch may influence the growth of desmoid tumors in addition to the Wnt pathway, which is canonically most closely linked to desmoids. Some studies have demonstrated elevated levels of Notch1, perhaps supporting this hypothesis. I would say that’s the extent of my knowledge. This has been written about extensively, but if there’s a tremendous interest see this paper from Shang and colleagues. 4
So, how did they study this drug anyways?
The DeFi trial was a randomized, double-blinded, placebo-controlled trial in which patients with progressing desmoid tumors were assigned 1:1 to nirogacestat and control.
The primary endpoint was progression free survival, which has been the standard for desmoid tumors. Given the behavior of desmoid tumors, this is appropriate. See my prior post as to why. There was a bevy of secondary endpoints which we’ll explore later.
In total, there were 142 patients that were assigned 1:1 into treatment and placebo arms. Recruitment took place in the US, Canada, and Europe. They needed to have progression by RECIST v 1.1 (20% growth) within the past 12 months. This would select for a particularly aggressive bunch of desmoid tumors. It is a little different from the sorafenib trial, which required a 10% increase in the last 6 months. Crossover was allowed at the time of progression, meaning patients who were failed by placebo were switched to nirogacestat. Patients were stratified by their location as well, which is an important consideration. The treatment group received oral nirogacestat 150mg or placebo twice daily taken continuously in a 28-day cycle.
Patients were recruited and randomized between May 2019 and August 2020.
One could argue that given the positivity of the Sorafenib study, placebo would not have been the standard at the time of trial initiation. This design was FDA vetted and, frankly, I would not expect a randomized study of sorafenib vs nirogacestat to be done—as important as the results might be. That said, this is not uncommon in oncology, and sorafenib is not FDA approved for desmoid tumor treatment, so there are valid arguments against it. Importantly, 61% of patients on this trial had received prior medical therapy, including about 25% who had gotten sorafenib.
The median age was 34 and the demographics were consistent with what we know about patients who get desmoid tumors. The study made an effort to point out that approximately 50% of patients were women of childbearing potential. We’ll talk about the significance of this later on, but suffice it to say that this is VERY IMPORTANT.
What were the results?
The data-cutoff was after a median follow-up of 15.9 months, which implies the average patient had been on the study for this long. There were 37 patients receiving placebo that had at least 20% growth of their desmoid tumor, compared with 12 patients on nirogacestat. Interestingly, the median progression free survival was in excess of a year, 15.1 months, for patients on placebo, while it had not yet been reached for those patients taking nirogacestat.
There was a large difference in the percentage of patients with a response (reduction in size of tumor by 30%). 41% of patients taking nirogacestat had response compared to only 8% of those on placebo. See the following figure.
Now, unfortunately, side effects were much more common for patients on the nirogacestat arm, with 55% reporting serious adverse events. The most common was diarrhea, followed by nausea, fatigue, and hypophosphatemia (low phosphorus). A side effect that was thankfully well studied, and I can elaborate more on in a separate post was ovarian dysfunction. This happened in 27 our of 36 (75%) women of childbearing potential receiving nirogacestat. The median time to onset was 8.9 weeks. This resolved in 20 of 27 women. This was unresolved for 5 of 27 women, but all had elected to continue nirogacestat. Needless to say, this means that for all people of childbearing potential, serious and transparent discussion should be held. It would likely also merit reproductive endocrinology consultation, which is often available at academic centers.
With the aforementioned, 21 patients of 70 (30%) prematurely discontinued the medication. This is a relatively high proportion and means that toxicity could eventually outweigh benefit for some patients.
In combination with more standard measures, patient reported outcomes were also tabulated using the GODDESS assessment, BPI-SF, and EORTC QLQ-C30 instruments. Not to discount these metrics, but also recognizing that I’ve already written in excess of 1400 words, I may explore these further in separate posts.
What do you think about nirogacestat (editorialize for me)?
Firstly, this is a rigorous and insightful trial that robustly evaluated the efficacy and safety of nirogacestat in a rare disease. It cannot be overstated how significant of an achievement this is. Furthermore, the authors have objectively sought to measure outcomes such as ovarian failure which have been underappreciated in other studies. This is the most impressive trial for desmoid patients yet.
It remains to be seen how this medication will be received by patients who will no doubt have questions about how it will fit into their lives, particularly those who are in their child bearing years. For this reason, continued longitudinal, and phase 4 study of this medication is definitely merited. Physicians administering this medication should be very open and transparent about the known adverse effects as well as not hesitate to enlist consultants such as fertility or reproductive endocrinology specialists.
The DeFi trial was a momentous undertaking which will shape the care of patients with desmoid tumors for years to come.
https://www.nejm.org/doi/full/10.1056/NEJMoa2210140
https://www.nejm.org/doi/full/10.1056/NEJMoa1805052
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30276-1/fulltext
https://pubmed.ncbi.nlm.nih.gov/26349011/