Obligatory - This is not medical advice
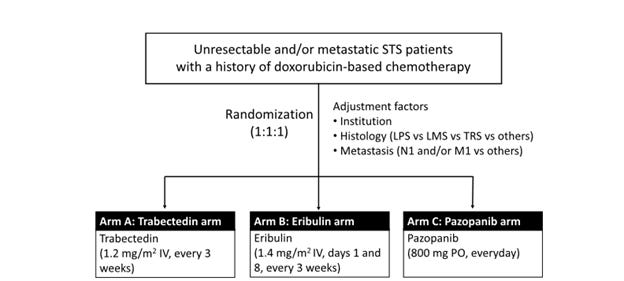
BMC Cancer just published the protocol for the 2ND-STEP study, JCOG1802, which is a randomized phase II trial of second-line treatment in patients with advanced soft tissue sarcoma being conducted out of 37 cancer centers in Japan. 1
The treatments being compared are:
1. Trabectedin 1.2mg/m2 over 24 hours once every 3 weeks2
2. Eribulin 1.4mg/m2 days 1, 8 of a 3 week cycle3
3. Pazopanib 800mg daily4
Any randomized undertaking within the sarcoma world is an impressive feat and I would say that the question being asked is an important one to answer. I’m going to skim through some of the details, but suffice it to say that the majority of inclusion criteria are acceptable, and, for a phase II study, progression free survival is a common endpoint. The gold standard, of course, continues to be overall survival and one can hope that this will be the primary endpoint in the phase 3 study that they allude to performing in the future.
Study Details:
Inclusion criteria are: age >16 years, unresectable or metastatic STS, diagnosis of STS other than Ewing sarcoma, embryonal/alveolar rhabdomyosarcoma, well-differentiated liposarcoma, and myxoid liposarcoma. Prior doxorubicin-based chemotherapy for soft tissue sarcoma, and ECOG 0-2.
Primary endpoint is progression free survival
Secondary Endpoints: overall survival, disease-control rate, response rate, safety
120 patients will be recruited and randomized
This regimen will ultimately be compared to gemcitabine and docetaxel
Within this paper they include what is a great summary of the data justifying the approvals of each of these agents, check it out below. This is a great reference and I will probably be sharing it with fellows who rotate through my clinic.
Let’s talk about strengths of this trial:
We will determine which, amongst standard options in second line, leads to the most time until progression of soft tissue sarcoma
The chosen agents are commonly used in the second line
The doses and frequency of each of these agents is appropriate (this had been an issue in other randomized Sarcoma trials before—EORTC 62931 I’m looking at you) 5.
The excluded histologic subtypes are reasonable
Problems:
Including liposarcoma in the pazopanib arm goes against the data from the PALETTE trial (ref). This may hamstring pazopanib.
I do not think that assuming gemcitabine and docetaxel would be the winner here is necessarily valid. We know that it is not superior to doxorubicin per the Geddis trial, but I think that it’s worth revisiting.
It may be worthwhile to further stratify histologies in this case, as the other category could be excessively broad.
Would prefer, so say we all, a primary endpoint of overall survival
CT scan frequency is a little strange (every 4 weeks until 16 weeks, then every 6 weeks). This is a little excessive by most standards and may shape the data.
In either case, I’m incredibly excited to see what the results will show. Let’s hope that enrollment moves quickly so that we can answer this question for our patients, and move into the phase III portion.
https://bmccancer.biomedcentral.com/articles/10.1186/s12885-023-10693-w
https://ascopubs.org/doi/10.1200/JCO.2015.62.4734
https://pubmed.ncbi.nlm.nih.gov/26874885/
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60651-5/fulltext
https://pubmed.ncbi.nlm.nih.gov/22954508/