Diagnosis and Treatment of Conventional Chondrosarcoma
A cartilagenous neoplasm
Obligatory—This is not medical advice
Elsewhere in this substack, we have reviewed sarcomas that arise from various types of cells-smooth muscle, endothelial, etc. Chondrosarcoma is a very mixed group of tumors that produce chondroid (or cartilaginous) matrix. There are different subtypes: conventional, dedifferentiated chondrosarcoma (treated similarly to osteosarcoma), mesenchymal chondrosarcoma (treated like Ewing sarcoma), and clear cell chondrosarcoma. 123 Herein, we will specifically focus on conventional chondrosarcoma, which is the most common histology.
Conventional Chondrosarcoma
Most chondrosarcoma diagnoses are of the conventional subtype (approximately 90%). Altogether, chondrosarcomas are approximately 20% of primary bone cancers with an incidence of 5 per 1M annually.4 In a recent evaluation of the SEER database, the most common age range for patients at diagnosis was 45-65 years with a mean of 53.5 Most common sites of origin were the limbs and axial skeleton. Outcomes were, as with other types of sarcomas, closely linked to tumor size as well as grade (see figure below). Note that Grade IV, as shown in the figure, indicates dedifferentiated chondrosarcoma.

On biopsy, pathologists see abundant cartilaginous matrix with chondrocytes (cartilage producing cells). Grade is determined by mitotic activity and atypia of those cells. Given the characteristic appearance and additional stains are often not required. Chondrosarcoma are, however, positive for S100 and D2-40.6 At time of diagnosis, imaging of the chest as well as appropriate evaluation of the local site is recommended.


Treatment
For patients with local, resectable disease, therapy is wide excision with negative margins. Data for radiation as well as systemic therapy is lacking in the adjuvant or neoadjuvant setting for conventional chondrosarcoma that has been completely resected. In settings of incomplete resection, multidisciplinary discussion is merited and adjuvant treatment may be considered. Nonetheless, most studies are small and/or retrospective limited in their interpretation. 78
In advanced disease, treatment selection relies on few small prospective trials, although studies are ongoing. Approximately 65% of chondrosarcomas may have mutations in isocitrate dehydrogenase 1 or 2 (IDH1, IDH2 respectively). This population was studied in a phase I study of ivosidenib in patients with IDH1 mutated advanced solid tumors.9
Study information:
This was a phase I multicenter open-label dose-escalation and expansion study.
The primary objective was to determined the maximum tolerated dose. Dosing of ivosidenib
Initial design was 3+310
21 patients with chondrosarcoma were enrolled
Multiple dose levels were administered to these patients
The maximum tolerated dose was not established in this study as there were ultimately no dose-limiting toxicities. Nonetheless, a dose of 500mg was selected based on pharmacodynamic analyses. Only one grade 3 adverse event (of those experienced by 12 patients) was deemed to be treatment related. There were no responses to this medication, although numerous patients had long term stable disease (see swimmer’s plot below).
A phase 2 prospective study of pazopanib for patients with advanced chondrosarcoma was published in 2020.11 The primary endpoint of this study was disease control rate at week 16, which is in essence asking what proportion of patients did not have growth of their disease by 30% after 4 months. The null hypothesis was that the this fraction would be less than 30%. Ultimately, the disease control rate as demonstrated by this study was 43%.
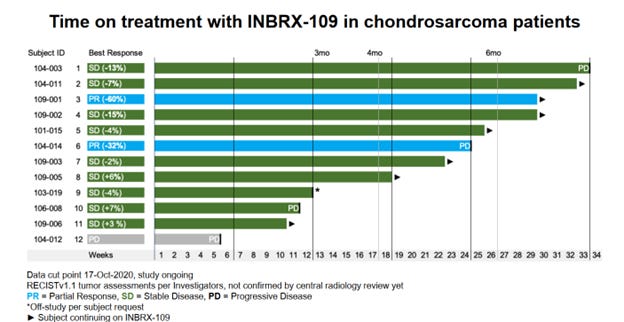
While these data provide some guidance about therapy, clearly additional options are needed, and trials are ongoing. One placebo-controlled study is evaluating INBRX-109 in comparison to control. 12 The primary outcome being assessed is progression free survival. Preliminary data from the phase I portion were presented at CTOS in 2020.
Conclusions
Chondrosarcoma is a malignant neoplasm of bone that represents approximately 30% of all primary bone sarcomas. Conventional chondrosarcoma is the most common subtype. Biopsy shows formation of a cartilaginous matrix. Treatment of local disease involves resection with negative margins. While tyrosine kinase inhibitors and medications targeting IDH1 may be used for patients with advanced disease, there remains a great need for more effective treatments for our patients. Trials are ongoing.
www.nccn.org
https://pubmed.ncbi.nlm.nih.gov/37174084/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4388572
https://europepmc.org/article/ppr/ppr356636
https://www.pathologyoutlines.com/topic/bonechondrosarcoma.html
https://onlinelibrary.wiley.com/doi/10.1002/jso.24368
https://pubmed.ncbi.nlm.nih.gov/24995550
https://ascopubs.org/doi/pdf/10.1200/JCO.19.02492?role=tab
https://ascopubs.org/doi/abs/10.1200/EDBK_319783?role=tab
https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.32515
https://clinicaltrials.gov/ct2/show/NCT04950075